According to current data of the WHO, gonorrhea is the third most common sexually transmitted infection (STI) worldwide. Unfortunately, there is no reliable epidemiological data from Germany as gonococcus infections do not fall under the mandatory reporting obligation. In addition to this, a global increase in resistance is observed. For the collection of valid data regarding the resistance situation in Germany, in 2013, project GORENET, in which Labor Krone takes part, was established.
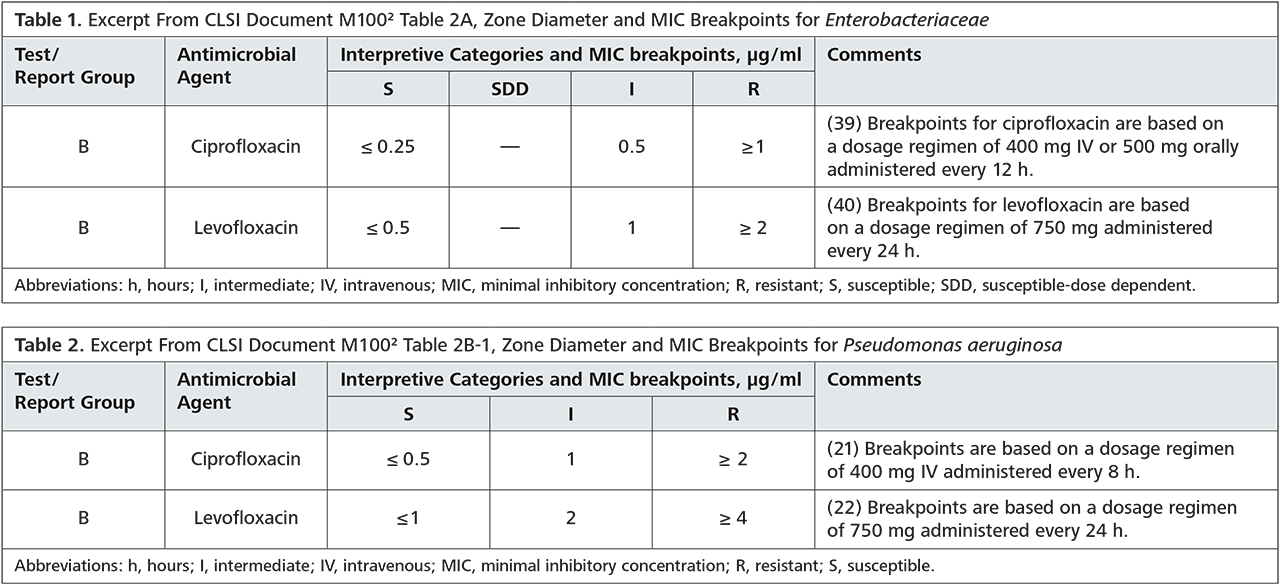
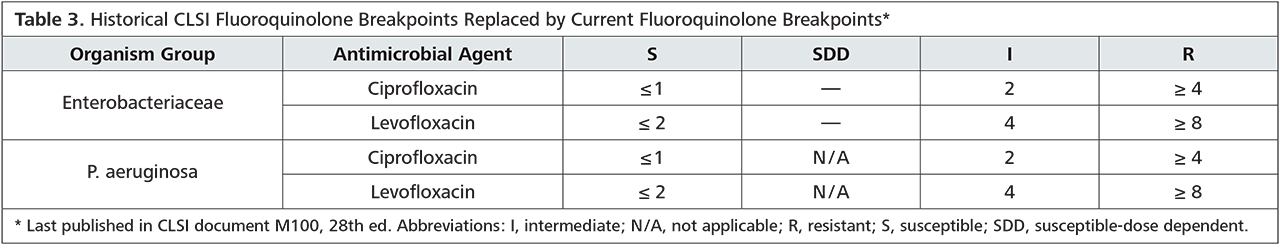
In view of the resistance situation in our region, we can follow the recommendations of the S2k-guidelines for empiric therapy: In our submissions, sensitivity for ceftriaxone is at 100 % (OWL alone at 100 %) and azithromycin at 88 % (OWL alone at 93 %). It should be noted, that administration of azithromycin in the empiric treatment rather aims at the co-infection with chlamydia.
An alternative ciprofloxacin therapy should only be used after previous testing, as the current resistance situation is insufficient for empiric treatment. The exact resistance situation is listed in the table below.
Conclusion: The guideline-oriented empiric therapy with ceftriaxone and azithromycin is recommended for this region. Prior to therapy commencement, swab diagnostics for gonococcus-PCR and gonococcus cultures (CAUTION: different swab kits!) should be carried out. Pathogen demonstration by PCR is more sensitive than a culture; however, resistance testing is only possible for culture-bred pathogens. Therapy monitoring can be in consideration of clinical aspects, if necessary, a swab for gonococcus culture 3 – 7 days after the end of therapy can be taken. Testing via PCR only makes sense a minimum of 2 weeks after the end of treatment, as otherwise avital pathogens could be detected. Partners should also be treated!
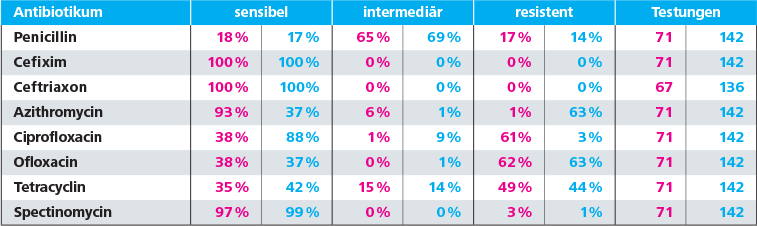
Resistances for Neisseria gonorrhoeae in OWL, or total number of returns from Labor Krone, period: 01.01.16 – 31.12.2018
Please do not hesitate to contact us if you have any further questions.
Department of microbiology
Dr. med. Patricia Wehmeier, Dipl.-Biol. Andreas Groß, telephone +49 5222 8076-0